
Unlocking Memory: How CA1 Neuron Dendrites Divide and Conquer Information Processing
Explore the distinct and vital roles of apical and basal dendrites in the hippocampus's learning and memory powerhouse.
CA1 pyramidal neurons, located in the hippocampus, are central players in the brain's ability to learn, form memories (especially episodic ones), and navigate space. These fascinating cells possess intricate branching structures called dendrites, which act as the neuron's primary receivers of information. Crucially, CA1 neurons have two distinct types of dendritic trees: apical and basal. Understanding their specialized functions is key to deciphering how the hippocampus processes information and supports complex cognitive tasks.
A colorful glimpse into the intricate network of hippocampal neurons, highlighting their complex structures like dendrites.
Highlights: Distinct Dendritic Roles
- Apical Dendrites: Specialize in integrating long-range inputs, particularly from the entorhinal cortex, crucial for spatial memory and sensory associations. They generate dendritic spikes to amplify signals.
- Basal Dendrites: Focus on processing local circuit information, receiving inputs from nearby CA3 neurons and interneurons, contributing to network excitability and local computations.
- Complementary Integration: Apical and basal dendrites work together, allowing CA1 neurons to act as sophisticated integrators, processing diverse inputs simultaneously for complex cognitive functions like learning and memory consolidation.
The Apical Dendrites: Integrating the Bigger Picture
Structure and Input Sources
Apical dendrites are characterized by a prominent, long trunk extending vertically from the apex (top) of the pyramidal cell body towards the outer layers of the hippocampus (stratum radiatum and stratum lacunosum-moleculare). This strategic positioning allows them to receive synaptic inputs from distant brain regions.
Key Input Pathways
- Entorhinal Cortex (Perforant Path): Direct cortical inputs, primarily targeting the distal tufts of the apical dendrites. These inputs convey highly processed sensory and spatial information crucial for forming contextual memories.
- Thalamus: Inputs from specific thalamic nuclei also converge on apical dendrites, contributing to the integration of information relevant to attention and sensory gating.
- CA3 Schaffer Collaterals: While primarily targeting basal dendrites, some Schaffer collateral inputs also reach the proximal apical dendrites.
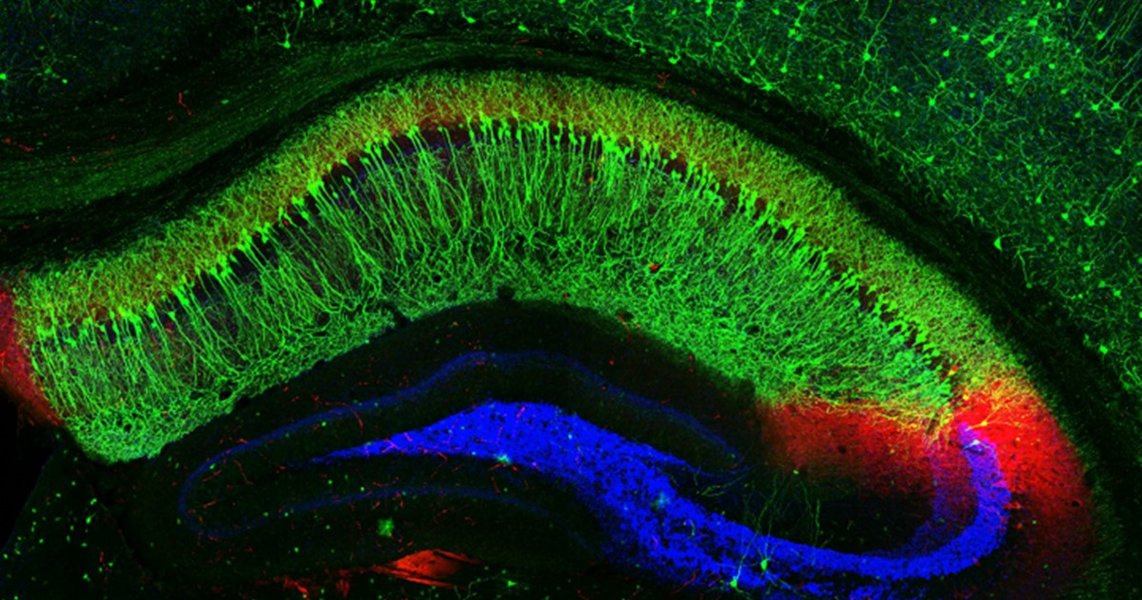
Artistic representation showing the layered structure of the hippocampus and the orientation of pyramidal neurons like those in CA1. Apical dendrites extend towards the outer layers.
Core Functions of Apical Dendrites
Apical dendrites are not just passive receivers; they actively process and transform incoming signals.
Synaptic Integration and Amplification
One of the most critical functions is the integration of thousands of synaptic inputs arriving at different locations and times. Apical dendrites, particularly the distal tufts, can generate regenerative electrical events known as dendritic spikes (dSpikes), often mediated by voltage-gated calcium or sodium channels. These dSpikes act as local amplifiers, boosting the impact of synchronized or strong distal inputs that might otherwise attenuate significantly before reaching the cell body (soma).
Synaptic Plasticity (LTP)
Apical dendrites are crucial sites for synaptic plasticity, particularly long-term potentiation (LTP), a cellular mechanism believed to underlie learning and memory. Dendritic spikes in the apical tree can provide the necessary depolarization to trigger LTP induction when paired with synaptic activity, effectively strengthening connections based on experience.
Modulating Excitatory/Inhibitory Balance
These dendrites receive both excitatory and inhibitory inputs. They play a vital role in balancing these signals, which is essential for precise information processing and preventing uncontrolled neuronal firing. Specific inhibitory interneurons target different segments of the apical dendrite, providing fine-tuned control.
State-Dependent Computation
The integrative properties of apical dendrites can change depending on the brain's overall state. For example, during theta oscillations (prominent during exploration and REM sleep), apical dendrites may process inputs differently than during sharp-wave ripples (associated with memory consolidation during rest). This adaptability allows the neuron to tailor its computations to the current behavioral context.
The Basal Dendrites: Processing Local Information
Structure and Input Sources
Basal dendrites radiate outwards from the base of the cell body, extending primarily within the stratum oriens. They are generally shorter and more branched than the main apical trunk.
Key Input Pathways
- CA3 Schaffer Collaterals: These are the primary excitatory inputs to basal dendrites, carrying information processed within the CA3 region of the hippocampus, crucial for pattern completion and recall.
- Local Interneurons: Various types of inhibitory interneurons synapse onto basal dendrites, providing crucial regulation of neuronal excitability and timing.
- Other CA1 Pyramidal Neurons: Recurrent connections between CA1 neurons can occur via basal dendrites, contributing to network dynamics.
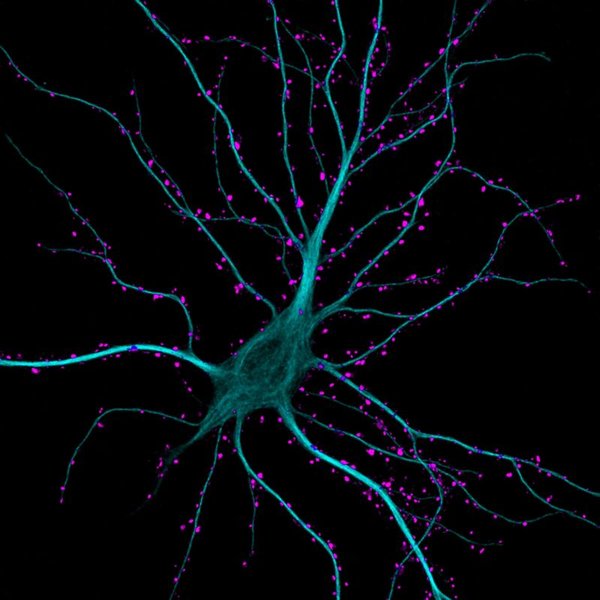
Micrograph showing a hippocampal neuron with dendrites receiving numerous excitatory synaptic contacts (highlighted in green). Both apical and basal dendrites receive such inputs.
Core Functions of Basal Dendrites
Basal dendrites specialize in processing information from within the local hippocampal circuit.
Integration of Proximal Inputs
Due to their proximity to the soma, inputs onto basal dendrites have a more direct and potentially rapid influence on action potential generation at the axon hillock. They integrate signals primarily from the CA3 region, contributing significantly to the neuron's overall firing pattern.
Local Circuit Processing
Basal dendrites are key players in processing recurrent information within the CA1 network and integrating feedback from local interneurons. This allows for fine-tuning of neuronal output based on the activity of the surrounding network.
Regulation of Neuronal Excitability
By integrating local excitatory and inhibitory inputs, basal dendrites significantly influence the overall excitability of the CA1 neuron, helping to control its firing threshold and response properties.
Contribution to Spatial Coding
While apical dendrites integrate contextual information, basal dendrites, processing inputs from CA3 (which itself contains place cells), contribute significantly to the formation and refinement of the spatial representations (cognitive maps) encoded by CA1 place cells.
Comparative Analysis: A Tale of Two Dendrites
Apical and basal dendrites possess distinct morphological, physiological, and functional characteristics, enabling CA1 pyramidal neurons to perform complex, multi-layered computations. While apical dendrites sample long-range, cortical information, basal dendrites integrate local, intra-hippocampal signals.
Functional Comparison Radar Chart
This chart visually compares the relative contributions and characteristics of apical and basal dendrites across several key functional dimensions. Higher values indicate a stronger association or capability. Note that these are comparative estimations based on typical functions.
Key Differences Summarized
The following table highlights the main distinctions between apical and basal dendrites in CA1 pyramidal neurons:
| Feature | Apical Dendrites | Basal Dendrites |
|---|---|---|
| Location | Extend from soma apex towards stratum radiatum & lacunosum-moleculare | Extend from soma base into stratum oriens |
| Primary Excitatory Input | Entorhinal Cortex (Perforant Path), Thalamus | CA3 (Schaffer Collaterals) |
| Input Range | Primarily distal, long-range | Primarily proximal, local circuit |
| Primary Function | Integration of contextual/spatial info, sensory associations, memory formation | Integration of local circuit activity, pattern completion input, excitability control |
| Dendritic Spikes | More prominent, especially Ca2+ spikes in distal tufts | Less prominent, mostly Na+ based near soma |
| Synaptic Plasticity Focus | LTP at Perforant Path synapses | LTP at Schaffer Collateral synapses |
| Morphology | Long main trunk, distal tuft | Shorter, more branched near soma |
Visualizing Dendritic Organization
Mindmap of CA1 Dendritic Functions
This mindmap provides a hierarchical overview of the key aspects of CA1 apical and basal dendrites, including their structure, main inputs, and primary functions discussed above.
Lacunosum-Moleculare"] id1a3["Long Trunk & Distal Tuft"] id1b["Inputs"] id1b1["Entorhinal Cortex (Distal)"] id1b2["Thalamus (Distal)"] id1b3["CA3 (Proximal Apical)"] id1c["Functions"] id1c1["Distal Input Integration"] id1c2["Dendritic Spikes (Ca2+/Na+)"] id1c3["Synaptic Plasticity (LTP)"] id1c4["Spatial/Contextual Memory"] id1c5["State-Dependent Computation"] id1c6["Sensory Association"] id2["Basal Dendrites"] id2a["Structure"] id2a1["Extends from Base"] id2a2["Stratum Oriens"] id2a3["Shorter, Highly Branched"] id2b["Inputs"] id2b1["CA3 Schaffer Collaterals (Proximal)"] id2b2["Local Interneurons"] id2b3["Other CA1 Neurons"] id2c["Functions"] id2c1["Proximal Input Integration"] id2c2["Local Circuit Processing"] id2c3["Excitability Regulation"] id2c4["Synaptic Plasticity (LTP)"] id2c5["Pattern Completion Input"] id2c6["Contribution to Place Fields"]
Dendritic Inhibition in Action
Structured Dendritic Inhibition
Understanding how inputs are integrated also requires considering inhibition. Research highlights that inhibitory inputs are not randomly distributed but are often targeted to specific dendritic domains, including apical and basal branches. This structured inhibition allows for sophisticated control over how excitatory inputs are summed and whether they trigger dendritic spikes or somatic action potentials. The video below provides an abstract discussing how structured dendritic inhibition supports branch-selective integration in CA1 pyramidal cells, further emphasizing the computational complexity occurring within these dendritic trees.
Frequently Asked Questions (FAQ)
What exactly are dendritic spikes?
Dendritic spikes (dSpikes) are regenerative electrical events, similar in principle to the action potentials generated near the soma, but they occur locally within the dendrites. They are typically triggered by strong or highly synchronized synaptic input. Unlike somatic action potentials which are usually all-or-none and primarily mediated by sodium channels, dendritic spikes can vary in amplitude and duration and can be mediated by sodium (Na+), calcium (Ca2+), or NMDA receptor channels. They function to amplify weak or distal synaptic inputs, enabling them to have a greater impact on the neuron's overall output, and play crucial roles in synaptic plasticity and local dendritic computation.
How do apical and basal dendrites contribute differently to memory?
Apical dendrites are crucial for encoding new episodic and spatial memories by integrating contextual information from the entorhinal cortex. Their ability to undergo significant plasticity (LTP) at these distal synapses allows for the storage of associations between different elements of an experience. Basal dendrites, receiving input primarily from CA3, are thought to be more involved in memory recall or pattern completion, using previously stored representations in CA3 to reconstruct memories based on partial cues. They also contribute to refining spatial representations (place fields).
What is Long-Term Potentiation (LTP) and why is it important here?
Long-Term Potentiation (LTP) is a persistent strengthening of synapses based on recent patterns of activity. It is widely considered a key cellular mechanism underlying learning and memory formation. In CA1 neurons, LTP can be induced at synapses on both apical (e.g., perforant path inputs) and basal (e.g., Schaffer collateral inputs) dendrites. The induction often requires coincident presynaptic activity (neurotransmitter release) and strong postsynaptic depolarization (which can be boosted by dendritic spikes). This process effectively makes communication across the synapse more efficient, 'storing' information by strengthening specific connections within the neural circuit.
Do apical and basal dendrites use different ion channels?
Yes, research indicates that the distribution and types of ion channels can differ between apical and basal dendrites, and even along the length of the apical dendrite. For example, certain types of voltage-gated calcium channels and potassium channels (like HCN channels contributing to 'sag' current) may be more densely expressed in distal apical dendrites compared to basal dendrites or the soma. These differences in ion channel composition contribute significantly to their distinct electrical properties, such as the propensity to generate different types of dendritic spikes, the way synaptic inputs summate, and how signals propagate towards the soma.
References
- On the Initiation and Propagation of Dendritic Spikes in CA1 Pyramidal Neurons - Journal of Neuroscience
- Principles of dendritic integration in CA1 pyramidal neurons - Janelia Research Campus
- The Dendrites of CA2 and CA1 Pyramidal Neurons Differentially Regulate Information Flow in the Cortico-Hippocampal Circuit - Journal of Neuroscience
-
Differentiation of Apical and Basal Dendrites in Pyramidal Cells and ... - PMC NCBI
- Pyramidal neurons: dendritic structure and synaptic integration - Nature Reviews Neuroscience
- Apical dendrite - Wikipedia
- Modeling apical and basal tree contribution to orientation selectivity ... - eLife Sciences
Recommended
Last updated April 12, 2025