
The Hidden Noble: Unveiling Krypton's Scientific Mysteries and Applications
A comprehensive exploration of element 36, from its shadowy discovery to its illuminating present-day technologies
Krypton Essentials: What Makes It Remarkable
- Noble Gas Inertness: Krypton's complete electron shell configuration makes it extraordinarily unreactive, enabling unique applications in lighting and insulation technology
- Rare Atmospheric Element: Comprising only about 1 part per million in Earth's atmosphere, krypton's scarcity contributes to its specialized industrial value
- Spectral Signature: Krypton produces distinctive green and yellow emission lines when electrically excited, making it invaluable for high-performance lighting applications
Introduction to Element 36: The Hidden Gas
Krypton (Kr) is a noble gas with atomic number 36, positioned in Group 18 of the periodic table between argon and xenon. Its name derives from the Greek word "kryptos," meaning "hidden" or "concealed" – an appropriate designation for an element that remained undiscovered until the late 19th century due to its extreme rarity in the Earth's atmosphere (approximately 1 part per million by volume).
As a noble gas, krypton exhibits the characteristic properties of Group 18 elements: colorless, odorless, tasteless, and exceptionally non-reactive under standard conditions. This chemical inertness stems from its stable electron configuration, with a full outer shell of electrons that minimizes reactivity with other elements.
Despite its scarcity and seeming reluctance to participate in chemical reactions, krypton plays significant roles in various technological applications, from high-performance lighting to window insulation, and from medical imaging to laser technology. Its unique physical and spectral properties have carved out specialized niches where krypton outperforms other elements and compounds.
Position in the Periodic Table
With an atomic number of 36, krypton sits in period 4, group 18 (the noble gases) of the periodic table. This position reflects its electron configuration: [Ar]3d¹⁰4s²4p⁶. This filled valence shell explains krypton's chemical stability and reluctance to form bonds with other elements under normal conditions.
This radar chart compares krypton with its noble gas neighbors argon and xenon across various properties and applications. Note that while krypton has similar reactivity to argon, it exhibits superior performance in lighting and insulation applications. Xenon shows slightly higher reactivity and medical applications potential, while krypton maintains a balanced profile of industrial utility.
The Discovery of Krypton: Unveiling a Hidden Element
The discovery of krypton represents one of the most methodical and deliberate searches for an element in scientific history. In 1898, British chemists Sir William Ramsay and Morris Travers were engaged in systematic investigations of the atmosphere, following Ramsay's earlier discovery of argon in 1894.
Their experimental approach was painstaking but precise: they progressively cooled and fractionally distilled liquid air, separating gases based on their different boiling points. After removing oxygen, nitrogen, and argon from their samples, they discovered a residual gas with unique spectral lines that had never been observed before.
The Spectral Signature
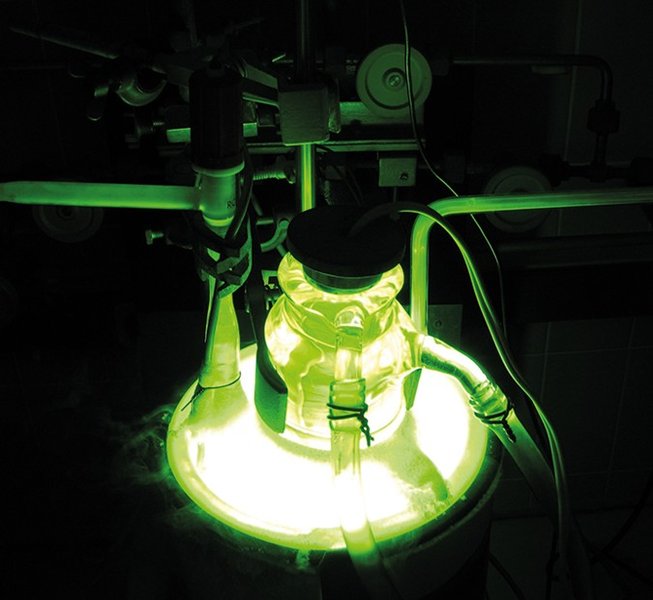
When Ramsay and Travers passed an electric current through the newly isolated gas, they observed a distinct spectrum with bright green and yellow emission lines—different from any known element. This spectroscopic evidence confirmed they had discovered a new element. The distinctive spectral signature would later become central to krypton's practical applications in lighting technology.
Etymology and Naming
Ramsay named the new element "krypton" from the Greek word "κρυπτός" (kryptos), meaning "hidden" or "concealed." This name was fitting for several reasons: krypton had remained hidden in the atmosphere despite decades of air analysis, it was present in extremely small quantities, and its chemical inertness made it difficult to detect through chemical reactions.
The discovery of krypton filled a predicted gap in the periodic table and helped validate Mendeleev's periodic law. It was the third noble gas to be discovered, after helium and argon, further establishing the existence of an entire group of inert elements that had not been anticipated in earlier versions of the periodic table.
Physical and Chemical Properties
Physical Properties: The Tangible Essence of Krypton
Krypton exists as a colorless, odorless, and tasteless monatomic gas under standard conditions. Its physical properties are fundamental to its various applications and provide insight into its behavior across different conditions.
| Property | Value | Significance |
|---|---|---|
| Atomic Number | 36 | Determines its position in periodic table and electron configuration |
| Atomic Mass | 83.798 amu | Approximately 3 times heavier than air, affecting its behavior in gas mixtures |
| Melting Point | -157.36°C (-251.25°F) | Relevant for cryogenic applications and separation processes |
| Boiling Point | -153.22°C (-243.80°F) | Close to melting point; narrow liquid phase range |
| Density at STP | 3.75 g/L | Higher than air, important for atmospheric stratification |
| Thermal Conductivity | Very low | Makes krypton excellent for insulation applications |
| Crystal Structure (solid) | Face-centered cubic | Typical of noble gases except helium |
Chemical Properties: The Reluctant Reactor
Krypton's chemical behavior is characterized by extreme inertness, though not absolute chemical inactivity. Its reluctance to form compounds stems from its complete valence electron shell, which confers exceptional stability.
Limited Reactivity
While generally unreactive, krypton can form compounds under extreme conditions, particularly with the highly electronegative element fluorine. The most significant krypton compound is krypton difluoride (KrF₂), first synthesized in 1963. This compound is a white crystalline solid that decomposes at room temperature, releasing krypton and fluorine.
The synthesis of KrF₂ typically requires high-energy conditions such as:
- Electric discharge through krypton-fluorine mixtures
- Ultraviolet irradiation of solid krypton in the presence of fluorine at very low temperatures
- Bombardment with high-energy radiation
The bond in KrF₂ is relatively weak compared to typical covalent bonds, reflecting krypton's reluctance to participate in chemical bonding. This limited reactivity highlights the fundamental nature of noble gases and their position at the boundary of chemical behavior.
Isotopes and Nuclear Properties
Krypton possesses a rich isotopic profile that contributes to its utility in various scientific applications, particularly in age dating and nuclear physics.
Natural Isotopes
Naturally occurring krypton consists of six stable isotopes: ⁸⁴Kr (the most abundant at approximately 57%), ⁸⁶Kr, ⁸²Kr, ⁸³Kr, ⁸⁰Kr, and ⁷⁸Kr. The distribution of these isotopes in natural krypton is as follows:
- ⁸⁴Kr: 57.0%
- ⁸⁶Kr: 17.3%
- ⁸²Kr: 11.6%
- ⁸³Kr: 11.5%
- ⁸⁰Kr: 2.3%
- ⁷⁸Kr: 0.4%
Radioactive Isotopes
Beyond the stable isotopes, krypton has several radioactive isotopes with varying half-lives. The most significant radioactive isotopes include:
⁸¹Kr: The Ancient Water Clock
⁸¹Kr has a half-life of approximately 230,000 years, making it an ideal isotope for dating ancient groundwater. This technique, known as krypton-81 dating, can reliably date water resources between 50,000 and 800,000 years old, filling an important gap between carbon-14 dating (effective up to about 50,000 years) and other dating methods for older materials.
⁸⁵Kr: The Anthropogenic Tracer
With a half-life of 10.76 years, ⁸⁵Kr is produced in nuclear reactions and has been released into the atmosphere through nuclear weapons testing and nuclear fuel reprocessing. This isotope serves as a tracer for atmospheric movement and mixing, providing valuable data for climate research and atmospheric science.
Occurrence and Extraction
Natural Occurrence
Krypton is one of the rarest elements in Earth's atmosphere, comprising approximately 1 part per million by volume. This scarcity contributes to its relative expense and specialized applications. While primarily found in the atmosphere, trace amounts of krypton can also be found in certain minerals, particularly those containing uranium, as krypton isotopes are produced during uranium decay.
Industrial Extraction
The commercial production of krypton relies on its extraction from the atmosphere through fractional distillation of liquefied air. This process involves several steps:
- Air is compressed and cooled until it liquefies
- The liquefied air undergoes fractional distillation to separate its components based on their different boiling points
- Nitrogen (bp: -195.8°C) and oxygen (bp: -183°C) are separated first
- The remaining fraction contains rare gases, including argon, krypton, and xenon
- Further distillation isolates krypton from other rare gases
- Final purification steps remove any remaining contaminants
This extraction process is energy-intensive and requires sophisticated equipment, contributing to the relatively high cost of pure krypton. Typically, krypton production is carried out as a side operation at large air separation plants primarily designed to produce oxygen and nitrogen for industrial applications.
Production Volumes and Economics
Global production of krypton is relatively modest compared to other industrial gases, with annual production estimated at several tens of metric tons. The limited supply and specialized production requirements contribute to krypton's relatively high price compared to more common gases like argon.
Applications: Illuminating the Hidden Element's Utility
Despite its rarity and chemical inertness, krypton has found numerous specialized applications across various industries, from lighting to window insulation to medical imaging.
Lighting Applications
Krypton's most widespread application is in the lighting industry, where its unique properties offer several advantages:
Incandescent Lighting
High-quality incandescent bulbs often use krypton (or a krypton-argon mixture) as the fill gas. Compared to cheaper nitrogen or argon fillings, krypton offers:
- Higher luminous efficacy due to reduced heat loss through the gas
- Extended filament life due to reduced tungsten evaporation
- Whiter light output with less yellowing over time
Fluorescent Lighting
Krypton is used in certain types of fluorescent lights, where its spectral emission characteristics contribute to improved color rendering and efficiency. Its distinct spectral lines (particularly in the green and yellow regions) can enhance the light quality when combined with appropriate phosphor coatings.
High-Intensity Discharge Lamps
Krypton is utilized in various specialized high-intensity discharge (HID) lamps, including those used in airport runway lighting, where reliable performance under extreme conditions is essential.
Window Insulation
Krypton's extremely low thermal conductivity makes it an excellent insulator in high-performance windows. In double or triple-glazed windows, the space between glass panes is filled with krypton or krypton-argon mixtures to reduce heat transfer and improve energy efficiency.
Benefits of krypton-filled windows include:
- Superior insulation compared to air or argon-filled windows
- Smaller gap requirements, allowing for thinner window assemblies
- Reduced condensation due to better thermal performance
- Enhanced sound insulation properties
While more expensive than argon-filled alternatives, krypton windows deliver superior performance in high-efficiency buildings where energy conservation is a priority.
Medical Applications
Krypton has several specialized medical applications, particularly in imaging and diagnostics:
Ventilation Imaging
Radioactive ⁸¹ᵐKr (a metastable isomer of krypton-81) is used in nuclear medicine for ventilation imaging of the lungs. This isotope emits gamma radiation that can be detected by specialized cameras, allowing physicians to assess lung function without exposing patients to long-lived radioactive materials.
Magnetic Resonance Imaging (MRI)
Hyperpolarized krypton isotopes are being researched as contrast agents for MRI, particularly for lung imaging. Krypton's properties allow for detailed visualization of lung tissue and air spaces, potentially offering advantages over conventional techniques.
Laser Technology
Krypton plays a key role in several laser technologies:
Krypton Ion Lasers
These lasers produce high-power, coherent light at several wavelengths, particularly in the green-yellow spectrum. They are used in scientific research, entertainment, and certain medical applications.
Krypton-Fluoride Excimer Lasers
KrF excimer lasers emit ultraviolet light at 248 nm and are used in semiconductor manufacturing (photolithography) and eye surgery (refractive procedures). These lasers rely on excited dimers (excimers) of krypton and fluorine that exist only in an excited state.
Krypton Compounds: Chemistry at the Edge
Although krypton is generally considered chemically inert, research since the 1960s has revealed a limited but fascinating chemistry, primarily with highly electronegative elements like fluorine and oxygen.
Krypton Difluoride (KrF₂)
The most significant krypton compound is krypton difluoride (KrF₂), first synthesized in 1963. This white crystalline solid represents one of the first conclusive demonstrations that noble gases could form chemical bonds under appropriate conditions.
Synthesis Methods
KrF₂ can be prepared through several methods:
- Electric discharge through a krypton-fluorine mixture at low temperatures
- Photochemical reaction between krypton and fluorine at -196°C (using UV light)
- Irradiation of solid krypton-fluorine mixtures with high-energy radiation
Properties and Structure
KrF₂ has a linear molecular structure (F-Kr-F) with bond lengths of approximately 1.89 Å. The bonding can be understood in terms of molecular orbital theory, with the krypton 4p orbitals participating in bonding. The compound is thermodynamically unstable at room temperature, decomposing to release krypton and fluorine.
Other Krypton Compounds
Research has revealed additional krypton compounds, though most are highly reactive and exist only under specific conditions:
Krypton Clathrates
Krypton can form clathrate compounds where krypton atoms are physically trapped within cage-like structures formed by other molecules, typically water. While not involving chemical bonds, these inclusion compounds represent another way krypton interacts with other substances.
Theoretical Compounds
Theoretical studies have predicted the possibility of other krypton compounds, including hydrides and oxides, though experimental verification remains challenging due to their expected instability.
Visualizing Krypton's Properties and Applications
Understanding Krypton Through Visual Elements
The following images showcase various aspects of krypton, from its appearance when excited by electricity to its practical applications in modern technology.


Understanding Krypton's Role in Science and Industry
This mindmap illustrates the interconnected nature of krypton's properties, history, and applications, providing a visual framework for understanding this fascinating element in context.
Krypton Compared to Other Noble Gases
Krypton occupies a middle position among the noble gases, between the lighter argon and the heavier xenon. This intermediate position gives krypton properties that make it uniquely suitable for certain applications where argon is insufficient and xenon would be unnecessarily expensive.
The Future of Krypton Research and Applications
Ongoing research into krypton continues to reveal new potential applications and deepen our understanding of this fascinating element.
Emerging Applications
Advanced Medical Imaging
Research into hyperpolarized krypton isotopes for enhanced MRI imaging shows promise for improved diagnosis of lung conditions. By manipulating the nuclear spin of krypton atoms, researchers can create powerful, non-invasive imaging agents that could revolutionize pulmonary diagnostics.
Next-Generation Insulation
Engineers are exploring composite insulation materials that incorporate krypton to achieve unprecedented thermal performance for applications in space technology, high-efficiency buildings, and cryogenic storage systems.
Environmental Monitoring
Krypton isotopes are increasingly valuable as atmospheric tracers for studying global circulation patterns and contamination spread, offering unique insights for climate research and environmental protection.
This video explores the unique properties of krypton and its significance in the periodic table. It provides valuable insights into what makes krypton such a fascinating element, from its discovery to its various applications in modern technology. The video helps visualize many of the concepts discussed in our exploration of this hidden noble gas.
Frequently Asked Questions About Krypton
References
- The world of krypton revisited - Nature Chemistry
- What Is Krypton Gas Used For? - WestAir
- Facts About Krypton - Live Science
- Krypton - Chemical Element - Britannica
- Krypton Facts - Atomic Number 36 Element Symbol Kr - Science Notes
- Krypton - Wikipedia
- Krypton - Chemistry Explained
Recommended Topics
Last updated April 7, 2025