
Electrophoresis: A Comprehensive Overview
Understanding the principles, types, and applications of electrophoresis in modern science
Key Takeaways
- Fundamental Principle: Electrophoresis separates charged molecules based on size and charge by applying an electric field.
- Variety of Techniques: Includes gel electrophoresis, capillary electrophoresis, isoelectric focusing, and more, each suited for specific applications.
- Wide Applications: Electrophoresis is essential in molecular biology, clinical diagnostics, forensic science, and biochemical research.
Introduction to Electrophoresis
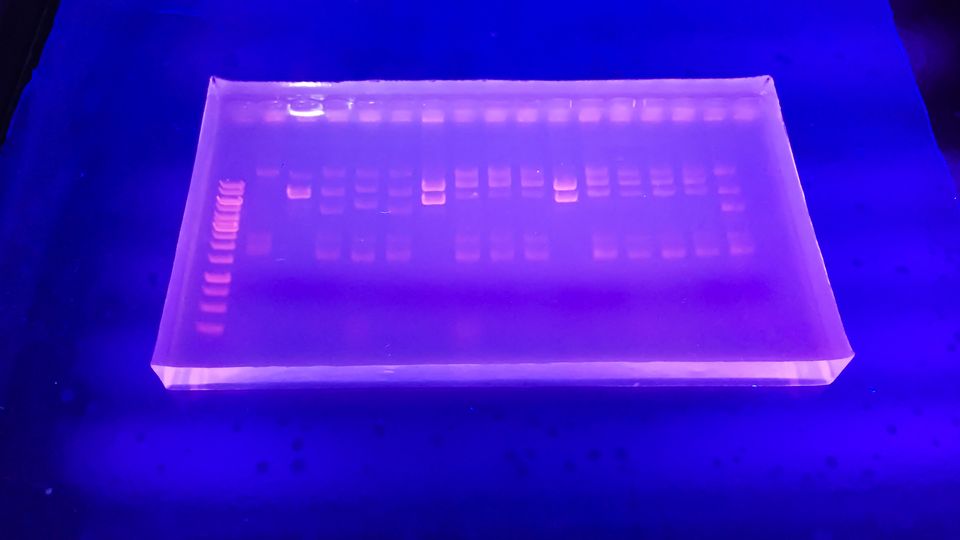
Electrophoresis is a pivotal laboratory technique used to separate and analyze charged biomolecules such as DNA, RNA, and proteins. By applying an electric field to a medium, electrophoresis allows the migration of these molecules based on their intrinsic electrical charge and size, facilitating various applications in molecular biology, biochemistry, and clinical diagnostics.
Principle of Electrophoresis
The core principle of electrophoresis involves the movement of charged particles through a conductive medium under the influence of an electric field. Positively charged particles (cations) migrate towards the negatively charged electrode (cathode), while negatively charged particles (anions) move towards the positively charged electrode (anode). Neutral molecules, lacking charge, remain largely stationary as they do not respond to the electric field. The rate of migration is influenced by factors such as the molecule’s charge, size, shape, the strength of the electric field, and the properties of the medium through which they are moving.
Types of Electrophoresis
1. Gel Electrophoresis
Gel electrophoresis is the most widely used form of electrophoresis, employing a gel matrix to facilitate the separation of molecules based on size and charge.
Agarose Gel Electrophoresis
Agarose gel electrophoresis is primarily utilized for the separation of DNA and RNA molecules. Agarose, derived from seaweed, forms a porous gel matrix through which nucleic acids migrate. The size-dependent migration allows for the distinction between larger and smaller molecules, with smaller fragments moving more rapidly through the gel.
Polyacrylamide Gel Electrophoresis (PAGE)
PAGE is typically used for protein separation and the analysis of smaller nucleic acids. Polyacrylamide gels possess tighter pores compared to agarose, enabling the separation of smaller molecules. Variations such as Sodium Dodecyl Sulfate PAGE (SDS-PAGE) are employed to denature proteins, ensuring separation based strictly on molecular weight rather than charge or shape.
Pulse Field Gel Electrophoresis
This technique is specialized for the separation of very large DNA molecules, which are not effectively separated by standard gel electrophoresis due to size constraints.
2. Capillary Electrophoresis
Capillary electrophoresis utilizes narrow capillary tubes filled with an electrolyte solution, offering high resolution and speed compared to traditional gel electrophoresis. It is widely used in analytical chemistry, forensic science, and pharmaceutical industries for the precise separation and analysis of ions and small molecules.
3. Isoelectric Focusing
Isoelectric focusing separates proteins based on their isoelectric point (pI), the pH at which they carry no net charge. By establishing a pH gradient within the medium, proteins migrate to the position where the pH equals their pI, effectively isolating them based on their unique pI values.
4. Affinity Electrophoresis
Affinity electrophoresis separates molecules based on their ability to form complexes with specific binding partners, such as protein-protein or protein-ligand interactions. This method is particularly useful for studying the interactions and binding affinities of biomolecules.
5. Moving Boundary Electrophoresis
Moving boundary electrophoresis encompasses techniques like isotachophoresis and immunoelectrophoresis, which involve the movement of boundaries between zones with different concentrations of analytes, facilitating the separation based on specific characteristics.
6. Routine (Zone) Electrophoresis
Commonly used in clinical laboratories, routine electrophoresis involves the separation of proteins on a slab gel, enabling the analysis of serum proteins for diagnostic purposes.
Applications of Electrophoresis
Electrophoresis is integral to numerous applications across various scientific domains:
Molecular Biology and Genetics
In molecular biology, electrophoresis is essential for analyzing DNA and RNA, such as verifying PCR products, conducting restriction enzyme analysis, and performing DNA fingerprinting. These techniques are crucial for cloning, sequencing, and genetic research.
Protein Analysis
In the analysis of proteins, electrophoresis aids in assessing protein purity, determining molecular weight, and examining post-translational modifications. SDS-PAGE, for instance, is extensively used to study protein expression and function.
Clinical Diagnostics
Electrophoretic techniques are employed in clinical diagnostics for detecting blood disorders. For example, hemoglobin electrophoresis is used to diagnose conditions like sickle cell anemia and various thalassemias by separating different hemoglobin variants.
Forensic Science
In forensic science, electrophoresis plays a pivotal role in DNA profiling and paternity testing, providing critical evidence for crime-solving and legal cases through the identification of unique genetic markers.
Pharmaceutical Research
Pharmaceutical research utilizes electrophoresis for analyzing drug purity, studying drug-protein interactions, and characterizing biologics, which is essential for the development and quality control of therapeutic agents.
Biochemical Research
Biochemists leverage electrophoresis to study enzyme kinetics, protein-protein interactions, and the structural analysis of biomolecules, contributing to a deeper understanding of biochemical pathways and mechanisms.
Practical Considerations in Electrophoresis
Successfully conducting electrophoresis requires careful attention to several practical aspects:
Buffer Systems
The choice of buffer is critical as it maintains the pH and ionic strength of the medium, which directly affects the migration of molecules. Common buffers include Tris-acetate-EDTA (TAE) and Tris-borate-EDTA (TBE) for agarose gels.
Voltage and Run Time
Optimizing the applied voltage and run time is essential to prevent overheating and band distortion. High voltage can accelerate migration but may compromise resolution, while low voltage may result in inadequate separation.
Gel Concentration
The concentration of the gel matrix should be adjusted based on the size of the molecules being separated. Higher gel concentrations provide better resolution for smaller fragments, whereas lower concentrations are suitable for larger molecules.
Interpretation and Troubleshooting
After electrophoresis, the separated molecules are visualized and interpreted to draw meaningful conclusions:
Visualization of Bands
Following electrophoresis, bands can be visualized using stains such as ethidium bromide for DNA or Coomassie Brilliant Blue for proteins. Comparing these bands to known standards or molecular weight markers allows for the determination of molecule sizes.
Troubleshooting Common Issues
Issues such as smearing or diffuse bands can arise from sample degradation, overloading, or problems with the gel matrix. Ensuring sample quality, optimal loading quantities, and proper gel preparation can mitigate these problems.
Electrophoresis Techniques Comparison
| Technique | Medium | Main Application | Advantages | Limitations |
|---|---|---|---|---|
| Agarose Gel Electrophoresis | Agarose Gel | DNA/RNA separation | Simple, suitable for large fragments | Lower resolution for small molecules |
| Polyacrylamide Gel Electrophoresis (PAGE) | Polyacrylamide Gel | Protein separation | High resolution for small proteins | Time-consuming preparation |
| Capillary Electrophoresis | Narrow Capillary Filled with Buffer | Small ions and molecules | High speed, high resolution | Requires specialized equipment |
| Isoelectric Focusing | pH Gradient Gel | Protein pI determination | Separation based on pI | Complex setup |
| SDS-PAGE | Polyacrylamide Gel with SDS | Protein molecular weight | Denatures proteins for uniform charge | Cannot analyze native protein structures |
Conclusion
Electrophoresis remains an indispensable tool in scientific research and clinical diagnostics, offering robust methods for the separation and analysis of charged biomolecules. Its versatility across various techniques allows for tailored applications, catering to the specific needs of molecular biology, biochemistry, forensic science, and medical diagnostics. By understanding its principles, types, and applications, scientists can effectively employ electrophoresis to advance knowledge and innovation in their respective fields.
References
Last updated February 13, 2025