
Unlocking Memory's Code: Why LTP Reigns Supreme in Cellular Studies
Discover how the persistent strengthening of brain connections provides a powerful window into learning and memory mechanisms.
Long-Term Potentiation (LTP) stands as a cornerstone in neuroscience for understanding how our brains learn and remember. It refers to a long-lasting enhancement in signal transmission between two neurons that results from stimulating them synchronously. This phenomenon, primarily studied in brain regions like the hippocampus crucial for memory formation, is widely recognized as a premier in vitro (laboratory-based) model for evaluating learning and memory at the cellular circuit level. But why does LTP hold such a prominent position in memory research?
Key Insights: LTP as a Memory Model
- Mimics Memory Properties: LTP exhibits characteristics essential for memory, such as persistence (lasting strengthening), specificity (only active synapses strengthen), and associativity (weak inputs strengthen if paired with strong ones).
- Shares Molecular Machinery: The cellular and molecular events triggering LTP, like NMDA receptor activation and protein synthesis, are also critical for memory consolidation in living organisms.
- Experimentally Testable Link: Manipulating LTP in vitro and in vivo directly impacts learning and memory behaviours, providing strong correlational evidence for its role.
The Foundation: Synaptic Plasticity and Hebb's Rule
Neurons That Fire Together, Wire Together
At its core, learning involves changing the connections, or synapses, between neurons. This ability of synapses to strengthen or weaken over time is called synaptic plasticity. LTP is a primary example of this plasticity in action. It represents a durable increase in synaptic efficacy following intense activation, providing a compelling cellular mechanism for how experiences can leave lasting traces in the brain.
Illustration depicting the complex network of synaptic connections where plasticity occurs.
LTP elegantly embodies the principle proposed by Donald Hebb in 1949, often summarized as "neurons that fire together, wire together." This Hebbian concept suggests that the simultaneous activation of connected neurons strengthens their synaptic link. LTP provides experimental validation for this idea:
- Specificity: LTP typically occurs only at the synapses that received the strong stimulation, not at inactive synapses on the same neuron. This mirrors how specific memories are encoded without affecting unrelated information.
- Associativity: A weak stimulus, normally insufficient to induce LTP, can potentiate its synapse if it occurs simultaneously with strong stimulation at another synapse on the same neuron. This mirrors associative learning, where we link related pieces of information.
- Cooperativity: LTP often requires the simultaneous activation of multiple afferent fibers to sufficiently depolarize the postsynaptic neuron, highlighting the cooperative nature of neural processing.
These properties make LTP an attractive candidate mechanism for how neural circuits adapt to store information, fulfilling key theoretical requirements for a memory substrate.
Delving Deeper: The Molecular Cascade of LTP
From Ion Flux to Lasting Change
The induction and maintenance of LTP involve a complex, yet elegant, sequence of molecular events within the synapse. Understanding this cascade is crucial to appreciating why LTP is such a powerful model.
Induction: The Initial Trigger
The process often begins with the activation of NMDA (N-methyl-D-aspartate) receptors, a special type of receptor for the neurotransmitter glutamate. Under normal, low-frequency synaptic transmission, the NMDA receptor channel is blocked by a magnesium ion (\(Mg^{2+}\)). However, when strong, high-frequency stimulation occurs (or when multiple inputs converge), the postsynaptic neuron becomes sufficiently depolarized. This depolarization dislodges the \(Mg^{2+}\) block, allowing calcium ions (\(Ca^{2+}\)) to flow into the postsynaptic neuron only when glutamate is also bound to the receptor. This requirement for both glutamate binding (presynaptic activity) and postsynaptic depolarization makes the NMDA receptor a 'coincidence detector,' perfectly suited for Hebbian learning.
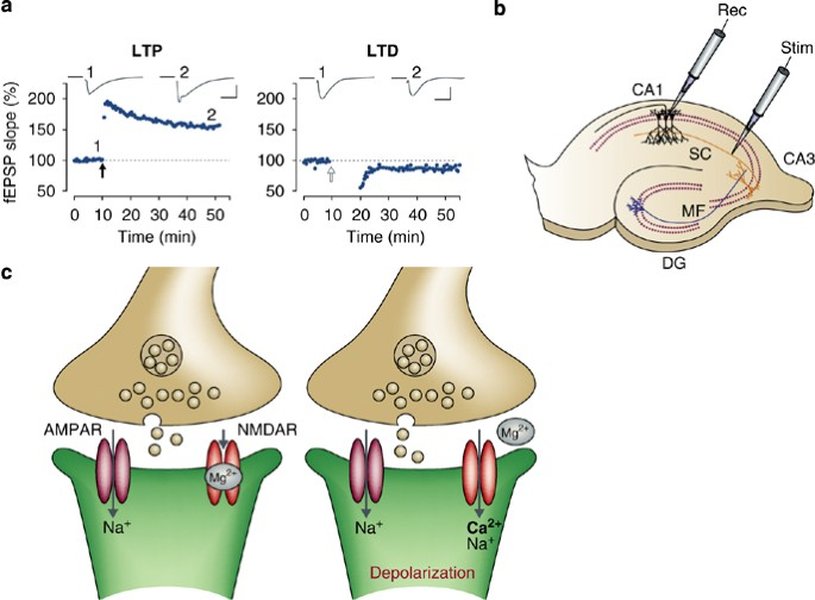
Diagram illustrating the roles of NMDA and AMPA receptors in synaptic transmission and plasticity.
Early Phase LTP (E-LTP): Rapid Enhancements
The influx of \(Ca^{2+}\) acts as a critical second messenger, initiating a cascade of intracellular signaling. Key players activated by \(Ca^{2+}\) include protein kinases, such as:
- Calcium/Calmodulin-dependent Protein Kinase II (CaMKII): This kinase is highly abundant at postsynaptic sites and undergoes autophosphorylation upon sustained \(Ca^{2+}\) elevation, making it persistently active even after calcium levels return to baseline. CaMKII phosphorylates existing AMPA receptors (another type of glutamate receptor responsible for most fast excitatory transmission), increasing their ion conductance.
- Protein Kinase C (PKC) and others: These kinases also contribute to phosphorylating target proteins.
A crucial outcome of this initial signaling is the trafficking of more AMPA receptors to the postsynaptic membrane. More AMPA receptors mean the synapse becomes more sensitive to glutamate released from the presynaptic terminal, thus strengthening the connection. E-LTP typically lasts for 1-3 hours and does not require new protein synthesis.
Late Phase LTP (L-LTP): Building for the Long Haul
For potentiation to last longer (many hours, days, or potentially longer), a more enduring process known as Late LTP (L-LTP) is required. L-LTP depends on changes in gene expression and the synthesis of new proteins. The initial signaling cascades, including kinases like PKA (Protein Kinase A) and MAPK/ERK (Mitogen-Activated Protein Kinase/Extracellular signal-Regulated Kinase), extend to the nucleus. Here, they activate transcription factors, most notably CREB (cAMP Response Element-Binding protein). Activated CREB promotes the transcription of genes whose protein products are necessary for stabilizing the synaptic changes and potentially creating new synaptic structures. These proteins can include receptors, signaling molecules, and structural proteins that remodel the synapse, making the strengthening more permanent. Research also points to the role of molecules like the long non-coding RNA Carip, which interacts with CaMKIIβ to regulate receptor phosphorylation, impacting LTP and spatial memory.
Visualizing the LTP Pathway
This mindmap outlines the key stages and molecular players involved in the transition from initial synaptic activation to long-lasting potentiation, highlighting the distinction between the early, transient phase and the later, more stable phase.
(Coincidence Detection)"] id1d1["Mg2+ Block Removal"] id1d2["Ca2+ Influx"] id2["Early Phase LTP (E-LTP)
(Hours, Protein Synthesis Independent)"] id2a["Ca2+ activates Kinases"] id2a1["CaMKII Activation/
Autophosphorylation"] id2a2["PKC Activation"] id2b["AMPA Receptor Modification"] id2b1["Phosphorylation
(Increased Conductance)"] id2b2["Trafficking/Insertion
into Postsynaptic Membrane"] id2c["Increased Synaptic Sensitivity"] id3["Late Phase LTP (L-LTP)
(Hours to Days+, Protein Synthesis Dependent)"] id3a["Persistent Kinase Activity
(e.g., PKA, MAPK/ERK)"] id3b["Signal to Nucleus"] id3c["Gene Expression Changes"] id3c1["CREB Activation"] id3c2["Transcription of
Target Genes"] id3d["New Protein Synthesis"] id3d1["Receptors"] id3d2["Signaling Molecules"] id3d3["Structural Proteins"] id3e["Synaptic Remodeling/
Growth"] id3f["Stable Synaptic Strengthening"]
The Hippocampus: A Key Arena for LTP and Memory
Where Learning Takes Shape
Much of the seminal research on LTP has been conducted in the hippocampus, a brain structure known to be essential for forming certain types of long-term memories, particularly spatial memory (like navigating a familiar environment) and episodic memory (memories of specific events). The highly organized structure of the hippocampus, particularly the pathway from the CA3 to the CA1 region (via Schaffer collaterals), allows researchers to reliably stimulate specific inputs and record the responses in postsynaptic neurons, often using in vitro hippocampal slice preparations.
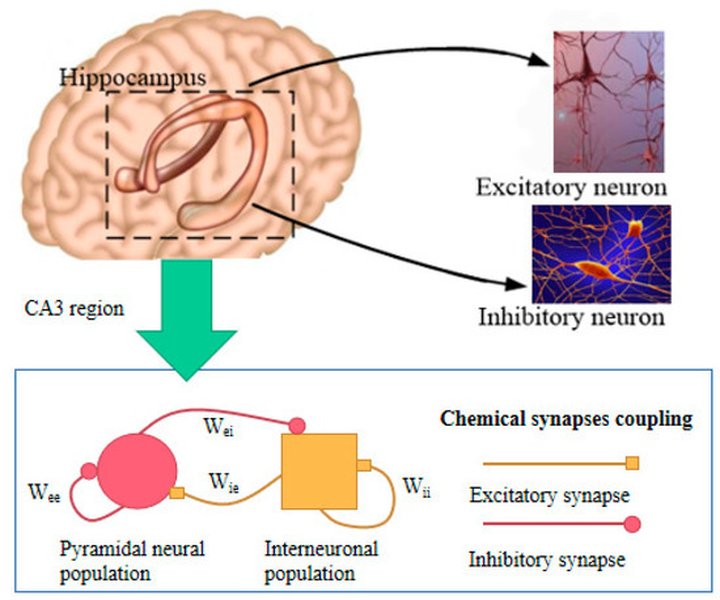
Schematic of the hippocampal circuit, highlighting the CA3-CA1 pathway frequently used in LTP studies.
The fact that LTP is so readily inducible and robust in this critical memory structure strongly supports its relevance to learning processes. Studies have shown that interventions impairing hippocampal LTP (like blocking NMDA receptors) also impair spatial learning tasks in animals. Conversely, conditions enhancing hippocampal function can correlate with enhanced LTP and learning.
Bridging the Gap: From Synapses to Behavior
Evidence Linking LTP to Learning and Memory
While LTP provides a compelling cellular mechanism, its status as a model for learning relies on evidence connecting it to actual behavioral changes. Several lines of research support this link:
- Pharmacological Blockade: Drugs that block NMDA receptors, and thus prevent the induction of many forms of LTP, also impair the ability of animals to learn new tasks, particularly those dependent on the hippocampus (e.g., spatial mazes).
- Genetic Manipulation: Genetically engineered mice lacking key molecules involved in LTP pathways (like specific kinase subunits or receptors) often show deficits in both LTP induction and learning tasks.
- LTP Saturation: If LTP is induced artificially to the point of saturation (synapses are maximally strengthened) in a relevant pathway, it can impair the animal's ability to learn new information that relies on that pathway. This suggests that the capacity for further potentiation is necessary for new learning.
- Correlation with Learning: Some studies show that the magnitude or persistence of LTP induced in an animal can correlate with its performance on certain learning tasks. Furthermore, learning experiences themselves can induce changes in synaptic strength that resemble LTP within the relevant brain circuits.
- Human Studies: While direct LTP measurement in humans is challenging, indirect evidence, such as correlations between genetic factors influencing synaptic plasticity (e.g., BDNF gene variants) and memory performance, supports the relevance of LTP-like mechanisms in human cognition.
Comparing LTP Properties to Memory Requirements
The following table highlights how key characteristics of LTP align with the functional requirements of a biological memory system:
| LTP Characteristic | Memory System Requirement | Explanation |
|---|---|---|
| Persistence | Memory Durability | LTP can last for hours in vitro, and potentially much longer in vivo (L-LTP), mirroring the long-lasting nature of memories. |
| Input Specificity | Memory Specificity | LTP strengthens only active synapses, allowing specific information/associations to be stored without disrupting others. |
| Associativity | Associative Learning | LTP allows weak inputs to be strengthened if paired with strong ones, providing a mechanism for linking related pieces of information. |
| Rapid Induction | Rapid Learning | LTP can be induced within seconds of appropriate stimulation, consistent with the brain's ability to learn quickly from experience. |
| Dependence on Correlated Activity | Hebbian Learning Rule | LTP often requires correlated pre- and postsynaptic activity (via NMDA receptors), matching theoretical models of how associations are formed. |
| Modifiability | Memory Updating/Forgetting | Synaptic strength potentiated by LTP can be reversed by processes like Long-Term Depression (LTD), allowing for flexibility and removal of outdated information. |
The Power of the Petri Dish: Experimental Advantages
Why In Vitro Studies are Crucial
A major reason LTP is widely used as a model is its experimental tractability, especially in in vitro preparations like brain slices. These preparations offer several advantages:
- Control: Researchers can precisely control the extracellular environment (e.g., ion concentrations, application of drugs) and the pattern of electrical stimulation applied to specific neural pathways.
- Accessibility: It allows direct electrophysiological recording from individual neurons to measure synaptic responses before and after stimulation, providing a clear readout of potentiation.
- Isolation: Studying circuits in a slice minimizes confounding variables from the rest of the brain or body, allowing focused investigation of synaptic mechanisms.
- Manipulation: It facilitates the application of pharmacological agents or genetic tools to dissect the roles of specific molecules (receptors, kinases, etc.) in the LTP process.
While in vivo studies are essential to confirm the behavioral relevance, the detailed mechanistic understanding of LTP has largely been built upon decades of rigorous in vitro research made possible by these advantages.
Visualizing Key Features of LTP
A Comparative Look at LTP Characteristics
This chart provides a conceptual visualization of several key features of LTP, rating their general prominence or importance based on current understanding. These characteristics collectively underpin LTP's suitability as a model for memory mechanisms. The ratings reflect general consensus rather than precise quantitative measures.
The chart highlights LTP's high experimental tractability and its strong exhibition of properties like specificity and persistence, crucial for a memory model. While the direct link to behavior is robust, it remains an area of intense ongoing research compared to the well-understood molecular mechanisms and in vitro characteristics.
Broader Implications and Context
Beyond the Basics: LTD, Disease, and Addiction
The Yin and Yang: Long-Term Depression (LTD)
LTP doesn't operate in isolation. Its counterpart, Long-Term Depression (LTD), involves a long-lasting decrease in synaptic efficacy. LTD is often induced by low-frequency stimulation and involves different calcium dynamics and downstream signaling pathways (often activating phosphatases instead of kinases). Both LTP and LTD are crucial for synaptic plasticity, allowing circuits to be fine-tuned, storing new information (LTP) while potentially weakening or removing less relevant connections (LTD). This balance is essential for preventing synaptic saturation and maintaining the capacity for new learning.
Relevance to Neurological Disorders and Addiction
Given its fundamental role in synaptic function and learning, disruptions in LTP mechanisms are implicated in various neurological and psychiatric conditions. Impaired LTP is observed in models of Alzheimer's disease, potentially contributing to memory loss. Conversely, aberrant synaptic plasticity, including alterations in LTP-like mechanisms in reward circuits (like the ventral tegmental area and nucleus accumbens), is thought to play a role in the development and persistence of addiction. Drugs of abuse can hijack these plasticity mechanisms, strengthening pathways associated with drug seeking and craving. Understanding LTP, therefore, offers potential therapeutic targets for these conditions.
Watch: LTP and Memory Formation Explained
This animation provides a clear visual overview of the Long-Term Potentiation process and its connection to how memories are believed to form and consolidate at the synaptic level within brain structures like the hippocampus.
Frequently Asked Questions about LTP
What's the difference between Early LTP (E-LTP) and Late LTP (L-LTP)?
Is LTP the only cellular mechanism for learning and memory?
How relevant is LTP studied in brain slices to memory in living animals or humans?
What are NMDA and AMPA receptors, and why are they important for LTP?
References
- The history of long-term potentiation as a memory mechanism - PubMed
- Properties of Long-Term Potentiation - Neuroscience - NCBI Bookshelf
- Long-Term Synaptic Potentiation - Neuroscience - NCBI Bookshelf
- Long-Term Potentiation and Memory - Physiological Reviews
-
Long-Term Potentiation - an overview - ScienceDirect Topics
- Long-term potentiation - Wikipedia
- Assessing the Relationship Between Long-Term Potentiation and Human Memory Performance - Frontiers in Human Neuroscience
Recommended Reading
Last updated April 13, 2025