
An In-Depth Look at Microfluidics
Exploring the Science and Applications of Fluid Manipulation at the Microscale
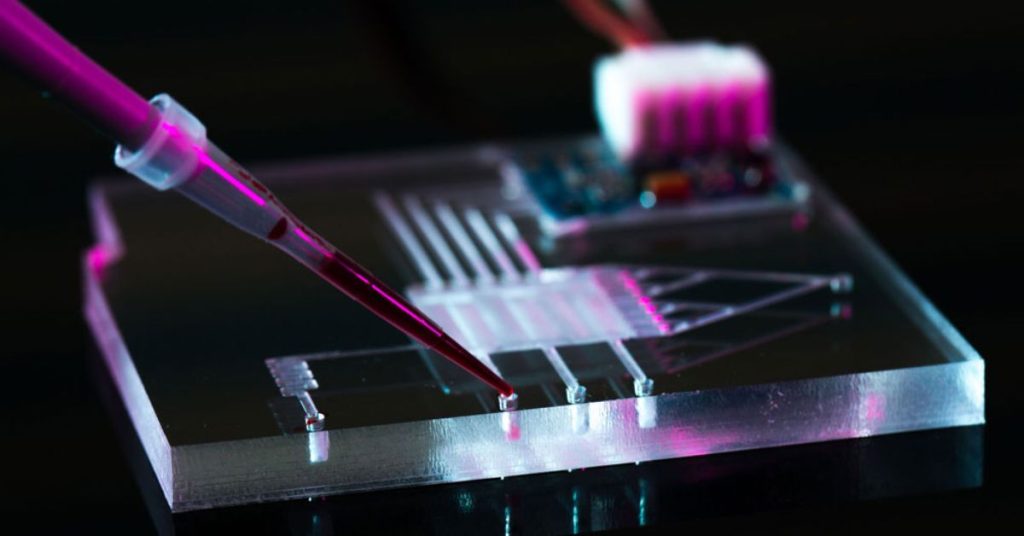
Key Highlights
- Definition and Scope: Microfluidics involves the precise manipulation of fluids in microscale channels, integrating principles from multiple scientific disciplines.
- Components and Techniques: It incorporates advanced techniques in fabrication, precise control through valves and pumps, and diverse materials including PDMS and silicon.
- Applications and Advantages: The technology is pivotal in biomedical diagnostics, chemical synthesis, environmental monitoring, and lab-on-a-chip innovations.
Understanding Microfluidics
Definition and Scope of Microfluidics
Microfluidics is the multidisciplinary field dedicated to the study and application of fluid behavior in microscale environments. The term generally refers to systems where fluids are manipulated within microchannels—channels that typically have dimensions ranging from tens to a few hundred micrometers. At these minuscule scales, phenomena that are negligible at larger scales, such as surface tension, capillary forces, and viscous effects, become dominant. This allows for extremely precise control over fluid dynamics and enables innovations across various scientific and engineering disciplines.
The field combines principles from physics, chemistry, biology, fluid dynamics, microelectronics, and materials science. Through its synergistic integration of these disciplines, microfluidics fosters the development of devices that can process and analyze samples in volumes ranging from microliters (\(10^{-6}\) liters) down to picoliters (\(10^{-12}\) liters). As a result, microfluidic systems are used to conduct detailed experiments with minimal resource consumption and reduced environmental impact.
Historical Context and Evolution
The evolution of microfluidics can be traced back to developments in microfabrication and semiconductor technologies. Initially, techniques refined for the production of microelectronic circuits were repurposed to create microchannels and microdevices. Over time, the improvement of materials such as glass, silicon, and various polymers (for example, PolyDimethylSiloxane or PDMS) enhanced the versatility of microfluidic devices. Today, microfluidics is at the heart of technologies like lab-on-a-chip and organ-on-a-chip systems, which streamline complex laboratory processes into compact, efficient devices.
Components and Fabrication Techniques
Core Components of Microfluidic Devices
A typical microfluidic device consists of several key elements designed to control and manipulate fluids:
Microchannels
Microchannels are the confined pathways through which fluids travel. Their dimensions often range from tens to hundreds of micrometers. They are designed to exploit microscale fluid dynamics and are fundamental in ensuring precise flow and reaction control.
Reservoirs
Reservoirs serve as the storage spaces or inlets and outlets for fluids within these devices. They ensure an even supply and collection of samples and reagents, playing an integral role in the overall operation of the system.
Valves and Pumps
Microfluidic valves regulate the flow between different compartments, and pumps generate the necessary pressure needed to drive the fluid through the channels. These components can be either passive—utilizing the natural properties of fluids—or active, involving external control mechanisms such as pneumatic or piezoelectric actuation.
Sensors and Detectors
Integrated sensors and detectors within microfluidic devices enable real-time monitoring of fluid properties such as temperature, pH, and chemical concentrations. They ensure that the processes at the microscale are precisely controlled and adjusted as needed.
Fabrication and Materials
Microfluidic devices can be fabricated using several methods, including photolithography, soft lithography, 3D printing, and etching techniques. The choice of fabrication technique typically depends on the desired resolution, material compatibility, and the specific application. For example:
| Fabrication Method | Advantages | Common Materials |
|---|---|---|
| Photolithography | High precision and resolution | Silicon, Glass |
| Soft Lithography | Flexible design and cost-effective | PDMS (PolyDimethylSiloxane) |
| 3D Printing | Rapid prototyping and adaptability | Various polymers |
| Etching Techniques | High-fidelity patterns | Glass, Silicon |
Among these, PDMS stands out as a popular choice because of its biocompatibility, optical transparency, and ease of use in soft lithography. PDMS-based devices have become the standard in many biomedical laboratories, enabling researchers to rapidly prototype and iterate their designs.
Applications and Advantages of Microfluidics
Biomedical and Clinical Applications
One of the most significant applications of microfluidics is in the biomedical field. By enabling precise control of fluids in miniaturized environments, these devices have revolutionized clinical diagnostics, drug discovery, and personalized medicine. Some key applications include:
Lab-on-a-Chip Devices
Lab-on-a-chip systems integrate multiple laboratory functions into a single miniaturized device. They are capable of performing complex analyses such as PCR (polymerase chain reaction), DNA sequencing, and chemical synthesis with high precision. These devices greatly reduce reagent consumption and processing time, making them cost-effective and efficient.
Point-of-Care Diagnostics
Microfluidic platforms are essential in developing portable diagnostic tools that can deliver rapid test results at the patient's bedside. This capability is crucial for managing diseases and expediting treatment decisions, enhancing the effectiveness of healthcare delivery.
Organ-on-a-Chip Technology
Organ-on-a-chip devices simulate the microarchitecture and functionality of human organs. These systems are invaluable for understanding disease mechanisms, testing new drugs, and studying tissue responses without the need for animal models.
Chemical Synthesis and Environmental Monitoring
In addition to biomedical applications, microfluidics has made significant inroads in chemical synthesis. Microreactors allow for highly controlled reactions with improved safety, better heat management, and increased yield. This controlled environment reduces waste and allows for the rapid testing of different reaction conditions.
Furthermore, microfluidic devices are increasingly utilized in environmental monitoring due to their capacity for high-throughput analysis and compact configurations. Biosensors integrated within these devices can detect and quantify pollutants, ensuring the rapid identification of contaminants in water, air, and soil samples.
Advantages of Using Microfluidic Systems
Microfluidic systems bring several advantages to research and industrial applications, including:
- Reduced Reagent Consumption: Minimized reaction volumes lead to significant cost savings and lower environmental impact.
- Faster Processing Times: The small scale of these devices allows for rapid mixing, reaction kinetics, and quick analytical results.
- High Precision and Control: Fine control over fluidic parameters enhances the accuracy and reliability of experiments.
- Integration and Automation: Multiple processes such as sample preparation, reaction, separation, and detection can be combined into a single chip, facilitating automated and high-throughput workflows.
- Portability: Compact design makes microfluidic devices ideal for point-of-care diagnostics and on-site environmental testing.
Emerging Trends and Future Directions
Integration with Digital Technologies
As microfluidic devices continue to evolve, their integration with digital technologies is paving the way for more sophisticated applications. The incorporation of sensors, wireless communication, and data analytics allows these systems to operate autonomously, providing real-time feedback and robust performance tracking. This integration is particularly important in personalized medicine where continuous monitoring can lead to tailored therapeutic interventions.
Advancements in Fabrication and Materials
Current research is focusing on improving fabrication techniques. Emerging methods such as 3D printing are enabling rapid prototyping and high-resolution fabrications that were previously unattainable with conventional methods. Additionally, advances in materials science are leading to the development of new polymers and composites that offer enhanced biocompatibility, durability, and functionality.
These innovations are expanding the possibilities of microfluidics, making it possible to design devices that are more complex and that can interface seamlessly with biological systems. This progress ultimately contributes to more effective diagnostic tools and better therapeutic devices.
Interdisciplinary Collaborations
The future of microfluidics is clearly interdisciplinary. Collaborations among engineers, biologists, chemists, and data scientists are resulting in groundbreaking innovations that push the envelope of what these devices can achieve. For instance, combining microfluidic technology with machine learning techniques allows for the real-time analysis of vast datasets generated during experiments, thereby refining diagnostic methods and enhancing the precision of chemical reactions.
Practical Considerations in Microfluidic Design
Device Design and Optimization
A successful microfluidic design requires thorough planning and optimization. Engineers must account for factors such as fluid properties, channel geometry, and fabrication constraints in their designs. Computational fluid dynamics (CFD) simulations are often used to model fluid behavior and optimize channel designs before fabrication begins. This iterative design process makes it possible to ensure that the final device operates efficiently and reliably.
Factors such as the Reynolds number, which characterizes the flow regime within the microchannels, are critical. At microscopic scales, fluid flows are typically laminar, meaning that the flow is smooth and predictable. This laminar flow condition is advantageous for applications requiring high precision and minimization of turbulence.
Challenges and Limitations
Despite the many benefits, microfluidic systems also face challenges. Fabrication defects, material incompatibilities, and integration of microscale components remain significant hurdles. Additionally, scaling up microfluidic devices for industrial applications poses its own set of challenges. Ensuring that the devices function consistently in varying environmental conditions, particularly when interfaced with biological samples, is another complexity that researchers are actively addressing.
Continuous improvements in design methodologies, materials, and fabrication processes are expected to mitigate these challenges, making microfluidic technologies even more reliable and versatile.
Analytical Techniques and Computational Approaches
Mathematical Modeling and Simulation
The use of mathematical models in microfluidics is essential for predicting and optimizing device performance. Researchers often use equations derived from fluid dynamics to model behaviors such as flow rates, mixing efficiency, and reaction kinetics. One common example is the application of the Navier-Stokes equations, simplified for low Reynolds number flows:
\( \displaystyle \nabla p = \mu \nabla^2 \textbf{u} \), where \(\textbf{u}\) is the fluid velocity, \(p\) is the pressure, and \(\mu\) represents the dynamic viscosity.
Such equations help predict how fluids will behave inside microchannels, enabling the design of devices that maximize performance and efficiency.
Data Analysis and Machine Learning Integration
Another growing trend is the integration of sophisticated data analysis techniques. Machine learning algorithms are increasingly being applied to the vast datasets generated by microfluidic experiments. This integration allows for improved control, predictive maintenance of devices, and optimized experimental setups, ultimately enhancing diagnostic accuracy and chemical synthesis throughput.
Future Prospects in Microfluidics
Personalized Medicine and Point-of-Care Technologies
The ongoing development in microfluidic technology holds tremendous promise for personalized medicine. By enabling rapid, precise analysis of biological specimens at the point-of-care, microfluidic devices can provide real-time feedback on a patient’s health status. This ongoing convergence of microfluidics with biotechnology is set to revolutionize medical diagnostics, operating on the principles of efficiency and minimal invasiveness.
Environmental and Industrial Applications
Beyond healthcare, microfluidics is making significant strides in industrial chemistry and environmental monitoring. For instance, microreactors facilitate the efficient synthesis of chemicals, often with superior safety profiles and higher yields than conventional methods. Similarly, microfluidic sensors are becoming essential in tracking environmental pollutants, offering rapid onsite analysis that traditional laboratory setups cannot match.
These applications illustrate the broad scope and transformative potential of microfluidics across multiple industries—from pharmaceuticals and diagnostics to sustainable chemical manufacturing and environmental stewardship.
References
- Microfluidics - Wikipedia
- Microfluidics: A Complete Overview - ALine Inc
- Microfluidics Overview - Fluigent
-
Fabrication and Applications of Microfluidic Devices: A Review - PMC
- General Overview of Microfluidics - Elveflow
Recommended Related Queries
Last updated March 15, 2025