
Unveiling Nano MXenes: A Revolution in 2D Materials Science
Explore the nanoscale world of MXenes—2D transition metal carbides and nitrides with unparalleled properties and transformative applications across diverse fields.
Key Insights into Nano MXenes
- Versatile 2D Materials: Nano MXenes are a rapidly expanding class of two-dimensional (2D) transition metal carbides, nitrides, and carbonitrides, first discovered in 2011, exhibiting remarkable properties at the nanoscale.
- Precision Synthesis: Primarily derived from layered MAX phase precursors, their synthesis often involves selective etching of the "A" layer, with ongoing advancements in both top-down (e.g., wet chemical etching, molten salt etching) and emerging bottom-up (e.g., CVD) approaches for enhanced control and safety.
- Multifunctional Properties: These nanomaterials boast exceptional metallic conductivity, high hydrophilicity due to surface terminations, large surface area, robust mechanical strength, and tunable optical and magnetic properties, making them highly attractive for diverse high-tech applications.
Nano MXenes represent a cutting-edge class of two-dimensional (2D) nanomaterials that have swiftly captivated the scientific community since their discovery in 2011. Comprising transition metal carbides, nitrides, or carbonitrides, these materials are characterized by their atomically thin, layered structures, typically with thicknesses under 1 nm and lateral dimensions ranging from nanometers to micrometers. The "nano" prefix underscores their critical nanoscale dimensions, which endow them with distinctive physical, chemical, and electronic properties not found in their bulk counterparts or other 2D materials like graphene.
Derived from their bulk, layered MAX phase precursors, MXenes are represented by the general formula \(M_{n+1}X_nT_x\). Here, \(M\) is an early transition metal (such as Ti, Nb, V, or Mo), \(X\) denotes carbon and/or nitrogen, \(n\) is an integer from 1 to 4 specifying the number of layers, and \(T_x\) signifies the surface terminations (e.g., hydroxyl (-OH), oxygen (-O), or fluorine (-F) groups) that naturally form during their synthesis. These surface terminations are pivotal, as they render MXenes hydrophilic, enabling their stable dispersion in aqueous solutions and facilitating further functionalization.
The Art and Science of Nano MXene Synthesis
The creation of nano MXenes is a sophisticated process that predominantly relies on top-down methods, though innovative bottom-up approaches are continually emerging. The goal is to selectively remove specific atomic layers from the MAX phase precursors while preserving the desired 2D MX layers, yielding high-quality nanoscale sheets.
(e.g., Ti₃AlC₂ to Ti₃C₂Tₓ)"] id1_1_2["HF-Forming Etchants
(e.g., LiF/HCl, NH₄HF₂, KF/HCl)"] id1_2["Molten Salt Etching
(e.g., LiF, NaF, KF)"] id1_3["Alkali-Assisted Hydrothermal Etching
(e.g., LiOH, LiCl)"] id1_4["Electrochemical Etching"] id1_5["Mechanical Delamination
(e.g., Ball-milling, Ultrasonication)"] id1_6["Fluorine-Free Methods
(Environmentally safer)"] id2["Bottom-Up Approaches"] id2_1["Chemical Vapor Deposition (CVD)"] id2_2["Template Methods"] id2_3["Plasma-Enhanced Pulsed Laser Deposition"] id2_4["Molecular Assembly"] id3["Post-Synthesis Processing"] id3_1["Intercalation
(e.g., TMAOH, organic cations)"] id3_2["Delamination
(Sonication, mechanical shaking)"]
The synthesis method profoundly influences the resulting MXenes' resistance to oxidation and hydrolysis, as well as their crucial electrical and physicochemical characteristics. Efforts are ongoing to develop fluorine-free and milder methods for MXene synthesis, which are highly desirable for improved performance, especially in applications like lithium-ion batteries and supercapacitors, and for enhanced environmental safety.
Top-Down Etching Methods: The Primary Route
The most common and established method involves the selective etching of the "A" group element layers from MAX phase precursors. This process typically utilizes aqueous solutions to remove elements like aluminum or silicon, leaving behind the layered MXene sheets. For instance, Ti₃AlC₂ is processed to yield Ti₃C₂Tₓ MXene through this method.
- **Wet-Chemical Etching:** This is the cornerstone method, frequently employing hydrofluoric (HF) acid or HF-forming mixtures (e.g., lithium fluoride (LiF) with hydrochloric acid (HCl), ammonium bifluoride (NH₄HF₂)). These etchants selectively dissolve the A-layers, simultaneously introducing the hydrophilic surface termination groups. The "minimally intensive layer delamination" (MILD) technique, specifically using LiF and HCl, is noted for producing high yields of few-layer MXenes with improved electrical conductivity and fewer defects.
- **Molten Salt Etching:** This technique employs molten salts like LiF, NaF, or KF at elevated temperatures to etch the A-layers, offering an alternative to traditional acid etching.
- **Alkali-Assisted Hydrothermal Etching:** Pathways like using LiOH and LiCl in aqueous solutions under hydrothermal conditions are also explored, capable of producing MXene dots and nanosheets, and even MXenes with doped heteroatoms.
- **Electrochemical Etching:** This method uses electrochemical processes in aqueous solutions to selectively remove the A-layer, offering a controlled and potentially scalable approach.
- **Mechanical Methods:** Ball-milling and mechanical-force-assisted ultrasonication have been employed to synthesize and delaminate MXenes, producing thinner and more uniform nanosheets.

An image showing the Hall Scattering Factor of MXene, illustrating its unique electronic properties.
Emerging Bottom-Up Approaches
While less prevalent than top-down methods, bottom-up strategies are gaining traction for their potential to synthesize MXenes with precise control over composition, structure, and fewer defects. These methods involve constructing MXenes atom-by-atom or layer-by-layer from molecular or atomic precursors.
- **Chemical Vapor Deposition (CVD):** This technique involves the reaction of gaseous precursors on a substrate to form thin films of MXenes, offering a route to high-purity, defect-free materials.
- **Template Methods:** These involve using a template to guide the assembly of precursors into specific 2D structures.
- **Plasma-Enhanced Pulsed Laser Deposition:** This advanced technique allows for the direct growth of ultrathin MXene layers with tailored properties.
After initial etching, post-synthesis steps like chemical intercalation (using agents such as tetramethylammonium hydroxide (TMAOH) or other organic cations) and delamination (often assisted by sonication or mechanical shaking) are crucial. These processes increase the interlayer spacing, facilitating the exfoliation into single or few-layer nano MXene flakes and creating stable nanoscale suspensions.
This video explains the synthesis of MXenes from MAX phase using the etching method, illustrating the fundamental process of deriving these 2D materials.
This video provides a clear visual and conceptual understanding of how MXenes are synthesized from their MAX phase precursors through the etching method. It highlights the transformation of bulk materials into atomically thin, two-dimensional structures, which is central to unlocking their unique properties and wide range of applications in nanotechnology.
Exceptional Properties of Nano MXenes
Nano MXenes exhibit a remarkable confluence of properties that positions them as highly attractive materials for advanced technologies. Their nanoscale dimensions amplify these attributes, enabling high performance in various environments.
Electronic and Electrical Prowess
- Metallic Conductivity: Unlike many other 2D ceramics, MXenes possess excellent metallic conductivity, with some optimized Ti₃C₂Tₓ variants exhibiting values up to 21,000 S/cm.
- Tunable Fermi Levels: Their Fermi levels can be precisely tuned through surface terminations or external stimuli, allowing for behavior that can range from metallic to semiconducting, crucial for electronic device integration.
- Adjustable Work Functions: This property supports their use in optoelectronics and various sensor technologies.
Hydrophilic and Surface Chemistry Versatility
- Hydrophilicity: The presence of hydroxyl, oxygen, and fluorine-based functional groups on their surfaces makes MXenes inherently hydrophilic. This allows for the preparation of stable dispersions in aqueous solutions, a significant advantage for solution-based processing.
- Tunable Surface Chemistry: The active termination groups enable precise surface modification through various strategies, including electrostatic adsorption, hydrogen bonding, and van der Waals forces, allowing for tailoring properties for specific applications.
Mechanical and Physical Attributes
- High Mechanical Strength: MXenes demonstrate strong mechanical properties, with mechanical strength comparable to or even surpassing graphene and carbon nanotubes in composites. For example, the tensile strength and modulus of epoxy/MXene composites can increase significantly.
- High Surface Area: Their thin, large lateral nanometer-sized morphology results in enormous specific surface areas, essential for applications in catalysis, energy storage, and sensing.
- Thermal Conductivity: MXenes possess high thermal conductivity, making them valuable in thermal management applications.
- Oxidative Stability: While initially a challenge, improved synthesis and storage conditions have significantly enhanced their longevity, though ongoing research continues to address this aspect.

An illustration of MXene as a component in advanced battery materials, highlighting its role in energy storage.
Optical and Magnetic Characteristics
- Outstanding Optical Properties: MXenes exhibit strong optical absorption, photothermal conversion capabilities, and tunable light-matter interactions. Their incorporation into nanocomposites can lead to synergistic optical absorption.
- Magnetic Properties: Some MXene compositions display fascinating magnetic properties, opening avenues for novel applications in spintronics and data storage.
Transformative Applications of Nano MXenes
The exceptional and tunable properties of nano MXenes translate into a vast array of applications across diverse fields, revolutionizing existing technologies and paving the way for new innovations.
This radar chart illustrates the relative strengths of MXenes across various critical performance indicators compared to other advanced 2D materials. While these are opinionated analyses rather than hard data, they reflect the general consensus on MXenes' outstanding capabilities. The chart highlights MXenes' leadership in electrical conductivity, high surface area, and versatile surface chemistry, making them highly competitive for diverse applications. Although oxidative stability has been a historical challenge, advancements in synthesis and storage are continually improving this aspect. Their biocompatibility is also a significant advantage, particularly for biomedical applications.
| Application Area | Key Advantages of Nano MXenes | Specific Examples |
|---|---|---|
| Energy Storage | High electrical conductivity, large surface area, excellent ion transport, tunable properties. | Electrodes in lithium-ion batteries, supercapacitors, sodium-ion batteries, and other energy storage components. |
| Environmental Remediation | High aspect ratio, extensive surface area, mechanical strength, chemical resilience, strong adsorption capabilities. | Water purification membranes (heavy metals, radionuclides, organic dyes), desalination membranes, electrocatalysts for water splitting. |
| Sensors | High sensitivity, tunable surface chemistry, excellent electronic properties, high surface area. | Gas sensors (NO₂, CO), fluorescent/optical sensors, electrochemical biosensors, wearable pressure/strain sensors, biochemical sensors. |
| Biomedical Applications | Biocompatibility, photothermal properties, enzyme immobilization platforms. | Photothermal therapy for cancer, theranostics, biosensors, drug delivery systems, dialysis, neural electrodes, enzyme immobilization for pesticide detection. |
| Electromagnetic Interference (EMI) Shielding | High electrical conductivity, layered structure, lightweight, flexible. | Flexible MXene films for EMI shielding in wearable electronics, communication devices, and aerospace. |
| Catalysis | High conductivity, high surface area, stability, synergistic effects with other materials. | Electrocatalysts for water splitting, reduction of nitrates, hybrid catalysts with enhanced activity. |
| Flexible Electronics & Composites | High electrical conductivity, mechanical enhancement, flexibility. | Conductive coatings, building blocks for combining with polymers, carbon nanotubes, graphene, or carbon dots (C-dots); components for triboelectric nanogenerators (TENGs). |
| Information Technology & Optoelectronics | Electronic and optical properties, potential for designing 2D materials on demand. | Advanced devices for information technology, LEDs, printable antennas, RFID tags. |
| Lubrication | Good mechanical, electrical, and chemical properties, low friction coefficient. | Lubrication applications for materials like Ti₃C₂ and Nb₂C MXenes. |
The field of MXenes is continuously expanding, with researchers estimating that millions of stable MXene compounds still await discovery, highlighting a very promising future for these materials.
Current Challenges and Future Frontiers
Despite their immense promise, the widespread adoption and full potential of nano MXenes face several challenges:
- Scalable and Controlled Synthesis: Achieving large-scale, cost-effective, and environmentally friendly synthesis of defect-free, monolayer nano MXenes remains a significant hurdle. Developing fluorine-free and milder etching methods is crucial for industrial scalability and safety.
- Oxidative Stability: Enhancing the long-term oxidative stability of MXenes is vital for their practical applications, as bare MXenes can be unstable and aggregate in certain environments.
- Surface Chemistry Control: Precisely controlling the surface chemistry and termination groups to tune functionality for specific applications requires advanced synthesis and functionalization techniques.
- Exploration of New Compositions: Fully exploring bottom-up synthesis methods and novel MAX phase precursors is essential to discover new MXene compositions and heterostructures with enhanced properties.
- Integration with Other Materials: Developing effective strategies for integrating MXenes with other materials to form high-performance composites while maintaining their unique properties is an ongoing area of research.
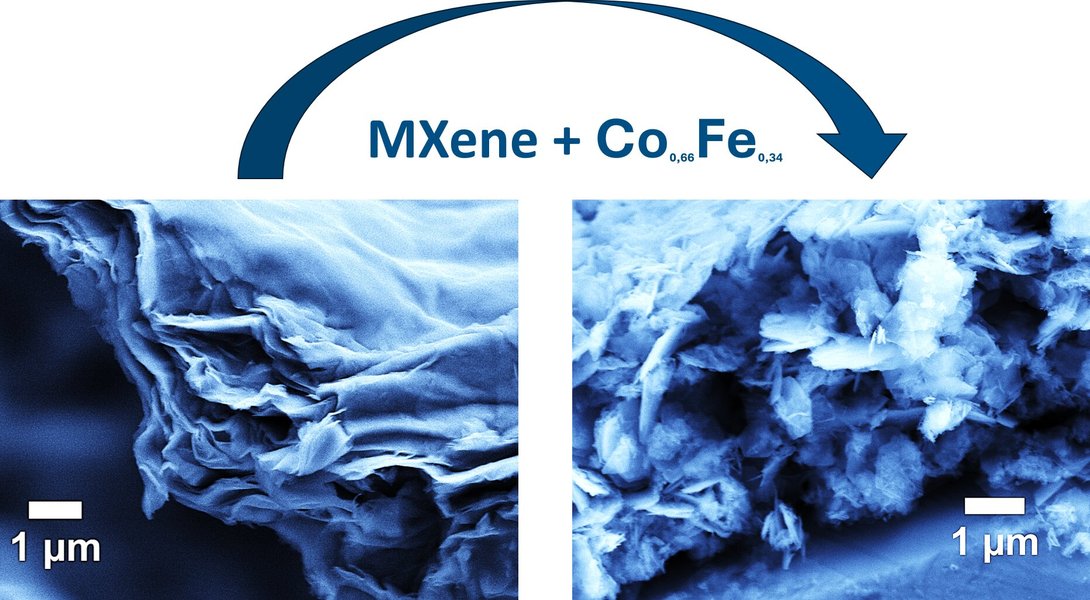
An illustration highlighting MXene's role as a catalyst in green hydrogen production, showcasing its environmental potential.
Frequently Asked Questions
Conclusion
Nano MXenes are at the forefront of materials science, offering an unprecedented combination of metallic conductivity, robust mechanical properties, and versatile surface chemistry. Their unique 2D architecture and tunable attributes make them ideal candidates for a broad spectrum of advanced technologies, from next-generation energy storage devices and environmental remediation systems to highly sensitive biosensors and flexible electronics. As synthesis techniques become more refined and scalable, particularly with the advent of fluorine-free and bottom-up methods, the potential for nano MXenes to revolutionize various industries will continue to expand. The ongoing research promises not only to unlock new compositions and properties but also to overcome current limitations, firmly establishing MXenes as essential building blocks for the future of nanotechnology.
Recommended Further Exploration
- Explore advanced synthesis techniques for fluorine-free MXenes to understand sustainable production.
- Investigate the mechanisms of MXene oxidation and methods for stability enhancement.
- Discover novel MXene-based composites designed for cutting-edge wearable electronics.
- Delve into computational modeling of MXene properties and predictions for new compositions.